Midkine home > About Midkine
Cancer and Midkine
Midkine expression is increased in many human carcinomas, such as esophageal, stomach, colon, pancreatic, thyroid, lung, breast, urinary bladder, uterine, ovarian, prostate and hepatocellular carcinomas, osteosarcoma, neuroblastoma and glioblastoma. This phenomenon is observed in about 80% of cases in many types of carcinomas (Fig.2).
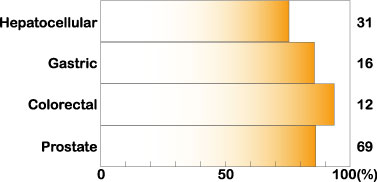
Fig.2
Increased expression of midkine in human carcinomas. Cited from Muramatsu, T., J. Biochem. 132, 359-371 (2002)
Furthermore, midkine expression is strongly increased in all cases of Wilmsユ tumor, in which loss of function of the tumor suppressor gene WT1 is frequently observed, and also in all cases of malignant nerve sheath tumor (MNST) caused by loss of the NF1 tumor suppressor gene. In neuroblastoma, urinary bladder carcinoma and gliobastoma, patients with tumors expressing a high level of midkine exhibit a worse prognosis than patients with tumors having a low level of midkine. Furthermore, overexpression of midkine is correlated with increased chemotherapy resistance in human gastric cancer cell lines.
The serum midkine level is increased in many cancer patients, and is planned to be used as a tumor marker. The midkine level frequently increases in the early stages of cancer progression, and is relatively high in cases of tumors with a poor prognosis, making the midkine level a promising tumor marker.
Midkine is thought to enhance tumor progression by promoting the survival, growth, migration and angiogenic activity of tumor cells. Antisense oligo DNA or siRNA directed at midkine suppresses growth of tumors in nude mice, opening the way to midkine-targeted cancer therapy. Furthermore, based on the preferential expression of midkine in tumors, the midkine promoter can be used to selectively express toxic genes in tumors. Animal experiments have been successful.
Inflammatory diseases and midkine
Midkine plays a central role in inflammation. For example, knockout mice deficient in the midkine gene poorly develop neointima, when the artery is damaged by ischemic shock. Renal damage after ischemia is also less extensive in the knockout mice than in wild-type mice. Furthermore, rheumatoid arthritis and multiple sclerosis in experimental models, and adhesions after surgery are much less severer in the knockout mice. Midkine promotes the migration of inflammatory leukocytes, namely macrophages and neutrophils. This migration is essential for inflammation, and a lack of midkine is considered to lead to prevention or a change of pathological status based on inflammation. Furthermore, midkine suppresses the development of regulatory T cells. Midkine is becoming a molecular target for the treatment or prevention of inflammatory diseases.
Prevention of cell death using midkine
Midkine has anti-apoptotic activity; the effect is best illustrated using embryonic neurons as target cells. Rentinal photoreceptor cells die after exposure to constant light in rats. Prior injection of midkine to the retina prevents the cell death. Temporary brain ischemia in gerbils leads to delayed neuronal death in the hippocampus. Prior delivery of midkine to the ventricle retards this process. Furthermore, midkine inhibits aboptosis of heart cells induced by ischemia. Thus, midkine is expected to prevent heart failure upon heart infarction.
Midkine is heavily deposited in senile plaques of patients with Alzheimerユs disease. Midkine binds to amyloid b-peptide and suppresses the cytotoxic activity. There is a possibility that midkine is produced to counteract the toxicity of amyloid β-peptide. Midkine enhances the survival of bovine embryos cultured in vitro. Furthermore, midkine has bactericidal activity and suppresses infection of HIV in target cells.
The anti-apoptotic and cell-protecting activites make midkine a promising therapeutic. However, the proinflammatory activity and protective activity of midkine should be carefully evaluated in each case.
Further activities of midkine
Midkine also play roles in regulation of blood pressure, bone remodeling and chondrogenesis. These activities are clinically important. As an example, midkine is a promising target for treatment of osteoporosis, since midkine enhances bone destruction.
Essentials of midkine
Protein
Midkine is a basic protein, essentialy composed of two domains held together by disulfide linkages. Each domain contains three anti-paralel b-sheets (Fig.3).
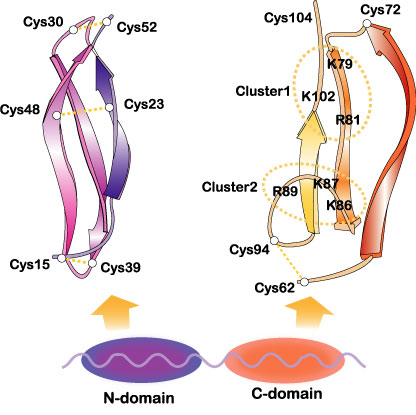
Fig.3
The domain organization of midkine and three dimensional structure of the domains. Two heparin-binding sites in the C-domain are encircled. Cited from Muramatsu, T., J. Biochem. 132, 359-371 (2002); Wiley Encyclopedia Mol. Med. pp2086-2088 (2002) [・2002, John Wiley & Sons]. This material is used by permission of John Wiley & Sons, Inc.
The more C-terminally located domain is usually responsible for midkine activity. Midkine is dimerized through the action of transglutaminase. Some midkine activity requires this dimerization. The more N-terminally located domain is required for the dimerization. Pleiotrophin[also called HB-GAM (heparin-binding growth-associeted molecule)]has 45 % sequence identity with midkine (Fig.4).
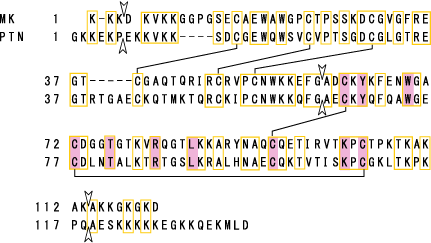
Fig.4
Protein structure of human midkine (MK). Amino acids conserved with pleiotrophin (PTN) are boxed. S-S linkages are shown by lines. Arrowheads show exon boundaries. Amino acids conserved also in Drosophila miple are shaded. Cited from Muramatsu, T., J. Biochem., 132, 359-371 (2002)
Midkine has been found in all vertebrata examined, namely from human to zebrafish. Zebrafish has two species of midkine. Although Drosophila lacks midkine, miple, and miple 2 molecules with repeating units homologous to the C-terminal half of both the midkine and pleiotrophin are present.
Gene
The human midkine gene is present in chromosome 11 band p.11.2, and is flanked by DGKz (diacyglycerokinase z gene) and CHRM4 (muscarnic acetylcholine receptor 4 gene) (Fig.5).
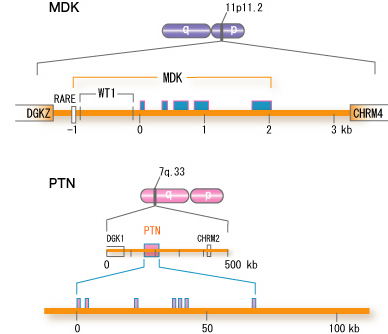
Fig.5
Structure of the human midkine gene (MDK). For comparison, the human pleiotrophin gene (PTN) is also shown. <,exon, RARE, retinoic acid responsive element; WT1, binding site for WT1 protein. There are two other variants in the first exon, not shown in the figure. cited from Muramatsu, T., J. Biochem. 132, 359-371 (2002)
The symbol for the human midkine gene is MDK. The mouse midkine gene (Mdk) is present on chromosome 2. In the upsteam of MDK, there is a retinoic acid responsive element, and midkine gene expression is induced by retinoic acid. Furthermore, the upstream region has a binding site for Wilmsユ tumor suppressor WT1. When the function of WT1 is lost, suppression does not take place, and midkine comes to be expressed. Although the pleiotrophin gene is located in a broader region of the human genome, the fundamental structure is similar.
Function and action mechanisms
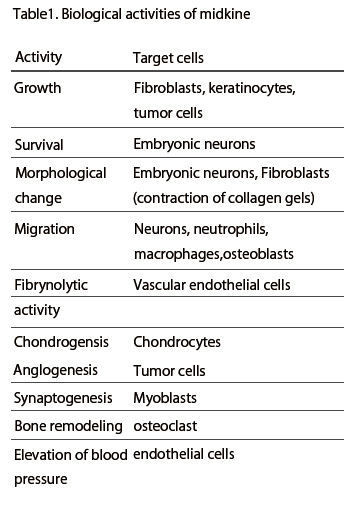
Midkine is most strongly expressed in midgestation. Epithelial tissues involved in epithelial mesenchymal interactions, nervous tissues during differentiation and mesenchymal tissues undergoing remodeling are the principal sites of expression. In the adult, midkine expression is restricted. Endothelial cells of blood vessels and mucus epithelium of certain organs are important sites of expression. When a tissue is injured, midkine expression is increased or newly induced. Midkine promotes the survival and migration of various cells, and also has many other activities ( Table 1). Using a blood vessel model, in which endothelial cells are layered on gels with smooth muscle cells, the complex mode of midkine action during epithelial mesenchymal interactions has been clarified (Fig.6).
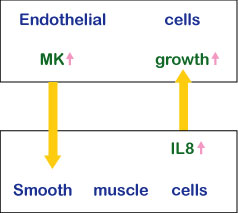
Fig.6
The action mechanism of midkine in epithelial mesenchymal interactions.
Among midkine receptors, receptor-type protein tyrosine phophafase z (PTP z) has been studied extensively. Midkine binds to the chondroitin sulfate portion with high affinity and to the protein portion with low affinity. In addition, low density lipoprotein receptor-related protein (LRP) and anaplastic leukemia kinase (ALK) have also been identified as receptors (Fig.7). Syndecans, a family of transmembrane heparan sulfate proteoglycans, can also participate in midkine signaling. Integrins and Notch-2 have been also found as components of the receptor. The midkine receptor is considered to be a molecular complex containing these proteins, the association of which is promoted by binding to midkine (Fig.8). The downstream signaling system contains PI3 kinase followed by ERK, and probably src and paxillin.
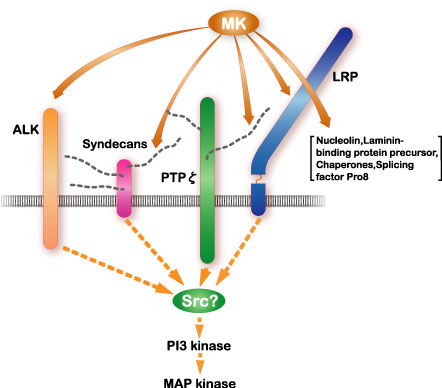
Fig.7
The signal receptor complex of midkine (MK) and the downstream signaling system. Cited from Muramatsu, T., J. Biochem., 132, 359-371 (2002)
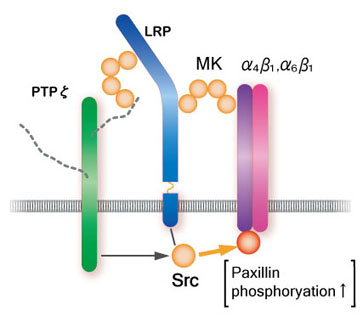
Fig.8
A model of the midkine receptor complex upon promotion of cell migration
Midkine binds to the oversulfated portion of heparan sulfate and chondroitin sulfate. The structure is shown in Fig.9
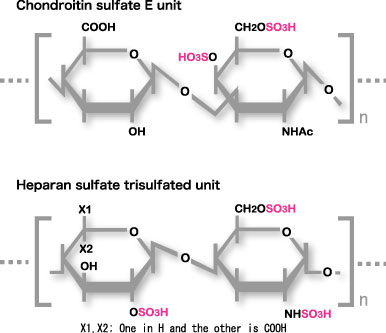
Fig.9
Carbohydrate structure required for strong binding to midkine. The Trisulfated structure in heparan sulfate and chondroitin sulfate E structure are shown.
Midkine is also incorporated into the cell and translocated to the nuclers. The survival promoting activity requires the nuclear translocation. LRP is involved in the uptake and nuclelin or laminin-binding protein precursor participates in the nuclear translocation.
Pleiotrophin and midkine
Pleiotrophin (PTN) exhibits about 50% sequence identity with midkine, The family of midkine and pleiotrophin may be called the miple family, since Drosophila has miples, which have a motif with similar degree of homology to the both factors. Mice deficient in only midkine or pleiotrophin exhibit relatively modest abnormalities under physiological conditions. However, mice doubly deficient in the both factors suffer from sever abnormalities such as low birth rate, low body weight (Fig.10), female infertility and auditory deficits. Thus midkine and pleiotrophin should play overlapping roles. Indeed, they share several in vitro activities such as promotion of migration, survival and process extension of neurons.
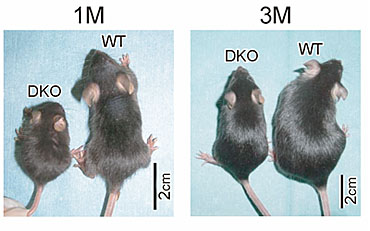
Fig.10
Mice doubly deficient in midkine and pleiotrophin (DKO) are smaller than wild-type mice. Muramatsu H. et al., Genes Cells, 11, 1405-1417 (2006)
However, midkine and pleiotrophin are evolutionally conserved as distinct molecules from fishes to humans. This implies that the two molecules of the miple family play some different roles. Indeed, so far only pleiotrophin has been reported to promote growth of human ES cells. Furthermore, pleiotrophin promotes differentiation of neural stem cells, while midkine promotes growth and survival of these cells. The difference in the sites of their expression is also important. Although both factors promote clustering of acetylcholone receptors, pleiotrophin serves as the factor expressed on the muscle, and midkine as the factor expressed on neurons.
Expression of the two molecules is increased upon tumorigenesis. However, increased expression of midkine is observed in a variety of tumors, while increase of pleiotrophin expression is more restricted. Nevertheless, overexpression in melanoma and myeloma has been reported only for pleiotrophin. Interestingly, neuroblastoma patients with strong midkine expression in the tumor exhibit worse prognosis, while those with strong pleiotrophin expression in the tumor show better prognosis.
Further information
More detailed information is available through reading review articles or visiting other home pages. The following are recommended.
1. Muramatsu, T. (2002) Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J. Biochem 132, 359-371.
[https://www.jstage.jst.go.jp/.../132/3/132_3_359/_article]
2. Muramatsu, T. (2002) Midkine in Wiley Encyclopedia of Molecular Medicine pp2086-2088. John Wiley & Sons., Inc. New York, USA.
3. Muramatsu, T. Chondroitin sulfate E in signaling of the growth factor midkine.
[http://www.glycoforum.gr.jp/science/glycogenes/09/09E.html]
4. Kurtz, A., Schulte, A. M., and Wellstein, A. (1995) Pleiotrophin and midkine in normal development and tumor biology. Crit. Rev. Oncol. 6, 151-177.
5. Locus link (http://www.ncbi.nlm.nih.gov/LocusLink/LocRpt.cgi?l=4192)
6. Kadomatsu, K., and Muramatsu, T. (2004) Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 204, 127-143.
7. Muramatsu T, Muramatsu H, Kaneda N, Sugahara K. (2003) Recognition of glycosaminoglycans by midkine. Methods Enzymol. 363, 365-376.
8. Muramatsu, T. (2010) Midkine, a heparin-binding cytokine with multiple roles in development, repair and diseases. Proc. Japan Acad. Ser. B 86, 410-425.[https://www.jstage.jst.go.jp/article/pjab/86/4/86_4_410/_article]
9. Muramatsu, T.(2011) Midkine: A promising molecule for drug development to treat diseases of the central nervous system. Curr. Pharm. Des. 17, 410-423. (Detailed description concerning drug development related to midkine) [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3267162/?tool=pubmed]
10. Erguven, M., Muramatsu, T. and Bilir, A. Eds,. (2012) Midkine: From Embryogenesis to Pathogenesis and Therapy , Springer [http://www.springer.com/biomed/book/978-94-007-4233-8].
11. British Journal of Pharmacology, midkine special issue (Vol 171, issue 4, 811-1067, 2014) (Many excellent reviews on midkine are present in this issue.) [http://www.springer.com/biomed/book/978-94-007-4233-8].
It is also possible to read original articles listed in Original articles as references. By searching Pub Med [http://www.ncbi.nlm.nih.gov/PubMed/] using midkine as a key word, more articles become available. The summary of an article listed in Original articles as references can be accessed using the PMID number written at the end of each reference.

 English
English Japanese
Japanese